The UK’s cannabis, CBD, and hemp sectors continue to mature, but regulatory expectations are rising just as quickly. Businesses that fail to keep pace face enforcement, stalled product launches, and damaged relationships with regulators. The Cannabis Trades Association supports companies across the entire supply chain, and one pattern is clear: most compliance failures are avoidable.
Below we explore the ten most common regulatory pitfalls that hold companies back. Understanding them is the first step to building a compliant, resilient, and scalable cannabis business.
1. Fuzzy Product Classification and Confused Claims
One of the fastest ways to attract regulatory attention is by misclassifying a product or mixing rules from different categories. Businesses often try to treat a product as a “food supplement”, but still imply medicinal benefits such as pain relief or anxiety treatment. This immediately triggers MHRA scrutiny.
At the same time, products containing THC or other scheduled cannabinoids may legally fall under Home Office control regardless of how the brand markets them.
A clear product classification at the start prevents MHRA enforcement, FSA questions, and Home Office licensing issues that many companies only discover when it is too late.
2. Weak Governance and No Responsible Person
Regulators expect clear leadership. When no senior individual is accountable for compliance and quality, problems follow quickly.
Without a Qualified Person, Responsible Person or QMS lead with genuine authority, key tasks drift into administrative corners. The Home Office expects a named licence holder with full accountability for controlled drugs. The FSA expects someone to own the Novel Food dossier and any changes to it.
When regulators sense that “nobody is really in charge”, trust collapses. Every application a company submits becomes harder to progress.
3. Retrofitting Quality Systems After the Fact
Many companies build facilities, purchase equipment and produce their first batches before creating their SOPs, quality manuals and records. This is a guaranteed path to failure.
A quality system must drive operations, not chase them. Inspectors will always test what happens in reality, not what is written on paper. When security procedures, QMS processes or controlled-drug registers exist only because the licence application required them, audits unravel quickly.
4. Poor Control of THC and Controlled Cannabinoids
Treating THC as a labelling detail is a critical mistake. THC is a controlled drug, and its handling is governed by criminal law.
Businesses regularly fall into trouble by misunderstanding exempt product conditions, storing controlled materials incorrectly, or failing to maintain accurate destruction and reconciliation records. Inconsistent THC levels in CBD products also risk Home Office attention and Novel Food non-compliance.
These issues lead to delayed licences, product seizures and long-term reputational harm.
5. Weak or Unrealistic Home Office Licence Applications
The Home Office licence is not a form to be filled out; it is a security and diversion-risk plan. Applications are often rejected because key details are vague or missing.
Common issues include incomplete site drawings, missing CCTV specifications, unclear access control systems and unplanned destruction workflows. When the Home Office cannot see how the company will prevent diversion, it cannot approve the licence.
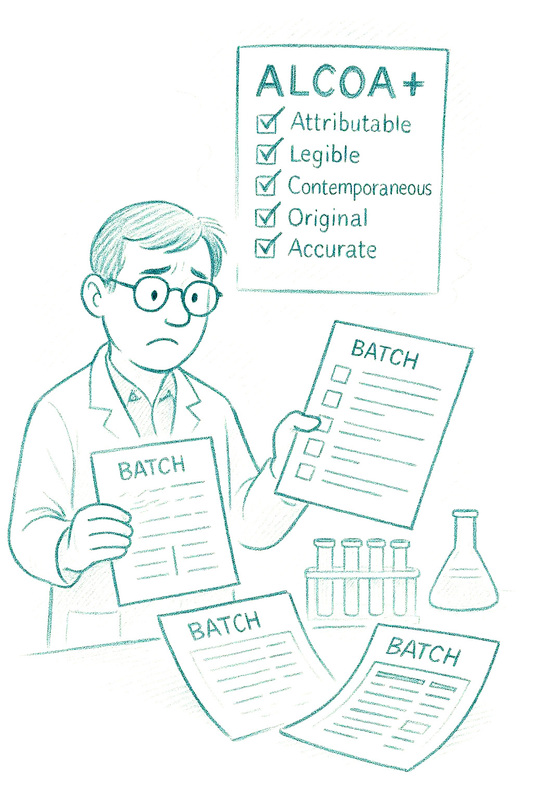
6. Neglecting Data Integrity and Documentation Discipline
Many businesses believe that regulators care mainly about good intentions. In reality, regulators care about data.
Without ALCOA+ principles, traceability collapses. Back-dated entries, shared logins and missing audit trails undermine even the best science or safety evidence. Novel Food dossiers fail when supporting documents are scattered across suppliers, contractors and outdated email chains.
If inspectors lose confidence in your data, they lose confidence in the business.

7. Underestimating the Novel Food Dossier
Novel Food is not an application; it is a scientific evidence package.
Incomplete ingredient characterisation, missing stability or contaminant data, mismatched supplier processes and unlinked intake assessments all lead to stalled or failing dossiers. Once submitted, the dossier must be kept updated. Any changes to extraction, formulation or supply chains must be formally assessed.
When a product drifts away from what was originally notified, compliance dissolves.
8. Vendor and Supply Chain Blind Spots
Many regulatory failures originate with poorly qualified suppliers. GMP certification alone is not enough. Regulators expect meaningful supplier oversight: audits, technical agreements, validated COAs and consistent processes that match your Novel Food dossier and marketing claims.
If a supplier cuts corners, the regulator does not blame them. They blame you.
9. Marketing Outrunning Compliance
Regulators watch what companies say in public.
Medicinal wording on “wellness” products triggers MHRA enforcement. Over-enthusiastic CBD marketing draws scrutiny from the FSA and trading standards. Casual or recreational references to THC can seriously damage a company’s credibility with the Home Office.
Marketing must not get ahead of regulatory strategy. Once trust is damaged, it is difficult to rebuild.
10. No Regulatory Roadmap or Budget
Compliance is a journey, not a checklist. Many businesses underestimate the time, cost and expertise needed for MHRA engagement, GMP inspections, Home Office licences or Novel Food submissions.
Under-resourcing compliance roles or failing to plan for rework causes projects to stall. Facilities are built but cannot operate. Products are developed but cannot be marketed. Investors lose confidence.
A phased regulatory roadmap is essential for sustainable growth.
How the CTA Can Help
Avoiding these pitfalls is not about being perfect. It is about building systems, governance and evidence that demonstrate professionalism, safety and accountability. The CTA provides guidance, training and industry support across MHRA, Home Office, GMP and Novel Food pathways.
If your company wants to reduce risks, strengthen compliance and move forward with confidence, the CTA is here to help.






