As the CBD industry expands, more businesses are diversifying their product ranges to include CBD foods, cosmetics, and even medicines. However, each of these product types is subject to different regulations and requirements in the UK and EU. To ensure compliance and success in the market, it’s essential to understand the legal landscape, labelling requirements, safety assessments, and application processes for each category.
In this step, we’ll break down the key considerations for developing and selling CBD foods, cosmetics, and medicines in the UK, highlighting the regulations you need to follow to stay compliant.
1. CBD Foods and Food Supplements
CBD foods and supplements, such as CBD oils, gummies, and drinks, are one of the fastest-growing segments of the market. However, due to their ingestion, they are subject to stricter regulations to ensure consumer safety.
1.1. Novel Foods Regulation
In the UK, CBD foods and supplements are regulated under the Novel Foods Regulation by the Food Standards Agency (FSA). Novel foods are those that have not been consumed to a significant degree in the UK or EU before May 1997.
What You Need to Know:
Novel Foods Application: All ingestible CBD products must be approved as a novel food by the FSA. Businesses must submit detailed safety assessments and dossiers showing that the CBD product is safe for consumption. You can apply as part of a group or as an individual business.
Deadline: Only products that were on sale before 13 February 2020 and have a pending novel foods application can remain on the market. Any new products must receive approval before they can be sold.
1.2. Labelling for CBD Foods
Nutritional Information: All CBD food products must have clear nutritional labelling, including the amount of CBD per serving.
Portion or Serving Size: Include recommended daily dosage and the Advisory Daily Intake (ADI) for CBD in the UK, which is advised at 10 mg/day.
Warnings: Labels should include warnings, such as “Not suitable for pregnant or breastfeeding women”, and directions for use.
No Health or Medical Claims: You cannot make unverified health claims on CBD food products. You can discuss general wellness benefits but avoid stating that CBD can “cure” or “treat” specific medical conditions.
1.3. Testing Requirements for CBD Foods
THC Testing: Ensure that your CBD food products contain less than 1mg of THC per finished product to comply with UK law.
Pesticides, Heavy Metals, and Microbial Testing: CBD food products must be tested for contaminants to ensure they are safe for human consumption.
2. CBD Cosmetics
CBD has gained popularity in the skincare and beauty industry, being used in creams, lotions, balms, and serums. However, like all cosmetic products, CBD cosmetics are subject to strict regulations to ensure safety and efficacy.
2.1. The UK Cosmetics Regulation
All cosmetics sold in the UK, including those containing CBD, must comply with the UK Cosmetics Regulation. This requires:
Cosmetic Product Safety Report (CPSR): Before you can sell any CBD cosmetics, the product must undergo a safety assessment by a qualified safety assessor. This ensures the product is safe for use on the skin and does not contain harmful levels of contaminants or allergens.
2.2. Labelling for CBD Cosmetics
Ingredients List: Use INCI (International Nomenclature of Cosmetic Ingredients) names when listing ingredients on the product label. The label must clearly state all ingredients in descending order of weight.
Warnings and Directions: Include any necessary warnings (e.g., “Avoid contact with eyes”) and instructions for use.
Claims: Avoid making medicinal or therapeutic claims that could classify your product as a medicine, rather than a cosmetic. For example, you can state that the product moisturises or soothes the skin, but avoid saying it “treats eczema.”
2.3. Packaging and Registration
Notification: CBD cosmetic products must be registered with the UK Submit Cosmetic Notification Portal before they are placed on the market. This provides the authorities with details about the product’s composition and safety assessment.
Cosmetic Packaging: Ensure that the packaging is compliant with UK standards, including tamper-evident seals and the name and address of the responsible person or business.
2.4. Testing Requirements for CBD Cosmetics
Stability Testing: CBD cosmetics must undergo stability testing to ensure that they maintain their quality over time and do not degrade before the stated expiry date.
Microbial Testing: Cosmetics should be tested for microbial contamination to ensure they are safe for use on the skin.
3. CBD Medicines
CBD products classified as medicines are subject to the strictest regulations. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) oversees the approval of medicinal CBD products. The process is lengthy and requires significant investment in research and clinical trials to demonstrate the product’s safety and efficacy.
3.1. What Classifies as a CBD Medicine?
A CBD product is considered a medicine if it is marketed for the treatment of specific medical conditions. This can include products intended to relieve pain, reduce inflammation, or treat anxiety. Any CBD product making medicinal claims must be authorised by the MHRA.
3.2. Licensing Requirements for CBD Medicines
To legally market a CBD product as a medicine in the UK, you must obtain a marketing authorisation from the MHRA. This process includes:
Clinical Trials: The product must undergo rigorous clinical testing to prove it is safe and effective for its intended use.
Good Manufacturing Practice (GMP): CBD medicines must be manufactured in facilities that meet GMP standards, ensuring high-quality production.
3.3. Labelling for CBD Medicines
Medicinal Information: Labels for CBD medicines must provide clear information about dosage, potential side effects, and any contraindications.
Packaging: The packaging must include patient information leaflets, explaining the use of the product, potential side effects, and instructions for use.
3.4. The Yellow Card Scheme
Once a CBD medicine is on the market, any adverse reactions must be reported to the MHRA’s Yellow Card Scheme. This helps monitor the safety of medicinal products and protect public health.
4. Key Differences Between CBD Foods, Cosmetics, and Medicines
Understanding the differences in the regulations and requirements for CBD foods, cosmetics, and medicines is critical for ensuring compliance and avoiding legal issues:
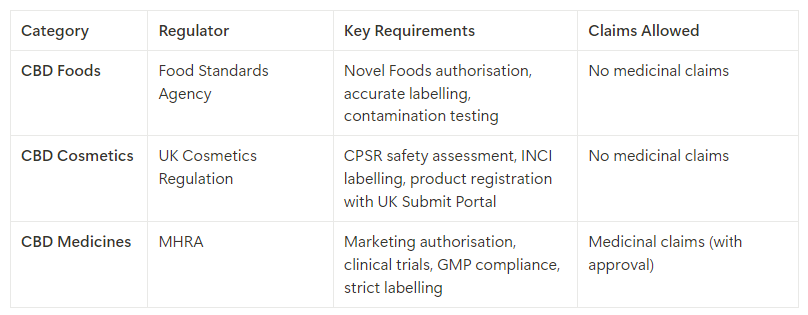
5. Best Practices for Product Development
Regardless of the category your CBD product falls under, there are several best practices to follow when developing and bringing a product to market:
Third-Party Testing: Use third-party laboratories to test for cannabinoids, THC levels, and contaminants. Share Certificates of Analysis (COAs) with customers to build trust.
Clear Labelling: Ensure all labels are accurate and compliant with UK regulations, avoiding any misleading claims.
Stay Updated on Regulations: The cannabis and CBD industry is evolving quickly. Stay informed about changes in UK and EU regulations to ensure ongoing compliance.






